Important Links
Appointments
If you think you are experiencing a medical emergency, call 9-1-1.
Doctors
Search by name, condition, location and more.
Clinics & Centers
Find your clinic or center for your appointment.
Contact Us
Contact the UAMS Winthrop P. Rockefeller Cancer Institute.
30+ Year Leukemia Survivor to Share Story at UAMS
The free event will be held at noon on Wednesday, April 17 in the Walton Auditorium on the 10th floor of the Cancer Institute. No registration is required.
Learn MoreNews & Announcements
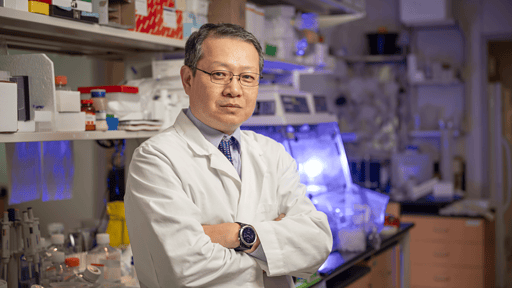
Distinguished Cancer Researcher, Yong Zhu, Ph.D., Joins UAMS Winthrop P. Rockefeller Cancer Institute Leadership
Prominent cancer epidemiologist, Yong Zhu, Ph.D., has joined the University of Arkansas for Medical Sciences (UAMS) Winthrop P. Rockefeller Cancer Institute as associate director for population science and translational science. He also holds an appointment as professor of epidemiology in the UAMS Fay W. Boozman College of Public Health. Zhu joins UAMS after serving for…
Read more
30+ Year Leukemia Survivor to Share Story at UAMS Winthrop P. Rockefeller Cancer Institute April 17
Mel Mann, a retired U.S. Army major and 32-year chronic myeloid leukemia (CML) survivor, will speak to cancer patients, caregivers and health care providers at the University of Arkansas for Medical Sciences (UAMS) Winthrop P. Rockefeller Cancer Institute grand rounds on Wednesday, April 17. The free event will be held at noon in the Walton…
Read more
UAMS Awarded $11.48 Million Federal Grant to Establish Center for Molecular Interactions in Cancer
The University of Arkansas for Medical Sciences (UAMS) Winthrop P. Rockefeller Cancer Institute received a five-year, $11.48 million federal grant to create the Center for Molecular Interactions in Cancer (CMIC). The grant was awarded by the National Institute of General Medical Sciences (NIGMS) Centers of Biomedical Research Excellence (COBRE) program. COBRE grants are awarded to…
Read more
Manojna Konda, M.D., and Vivek Yadala, M.D., Join UAMS Baptist Health Cancer Network
Oncologists Manojna Konda, M.D., and Vivek Yadala, M.D., have joined the University of Arkansas for Medical Sciences (UAMS) to support the expansion of UAMS cancer services to Baptist Health locations. Konda is a medical oncologist treating patients at the UAMS Baptist Health Cancer Center at Baptist Health Medical Center in Little Rock. She is a…
Read moreWant to read more stories like these?