- Fenghuang (Frank) Zhan, M.D., Ph.D. Moves Myeloma Research forward with Grant Funding
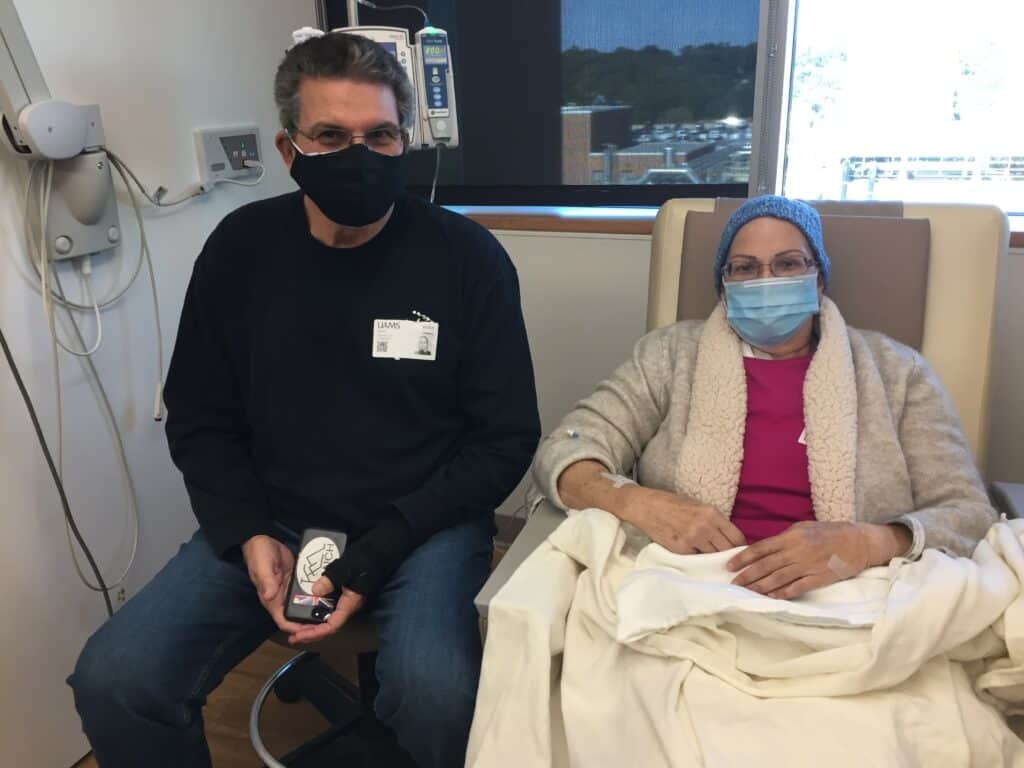
- First Myeloma Center Patient Receives Revolutionary New Therapy, Responds Well
- Patients’ Care at Myeloma Center Continues Despite Pandemic
- Partners in Care
- Retired UAMS HR Chief Shares Myeloma Journey
Through Blog - UAMS Raises $15 Million in Quest for National
Cancer Institute Designation - Physician, Pioneering Researcher Join UAMS Myeloma Center / Researcher Returns
- CAR T-CELL Therapy Underscores a New Era in
Myeloma Treatment - Grateful Husband Donates in Honor of Late Wife
- Publications
- Patient Travels Nearly 4,000 Miles for his Care;
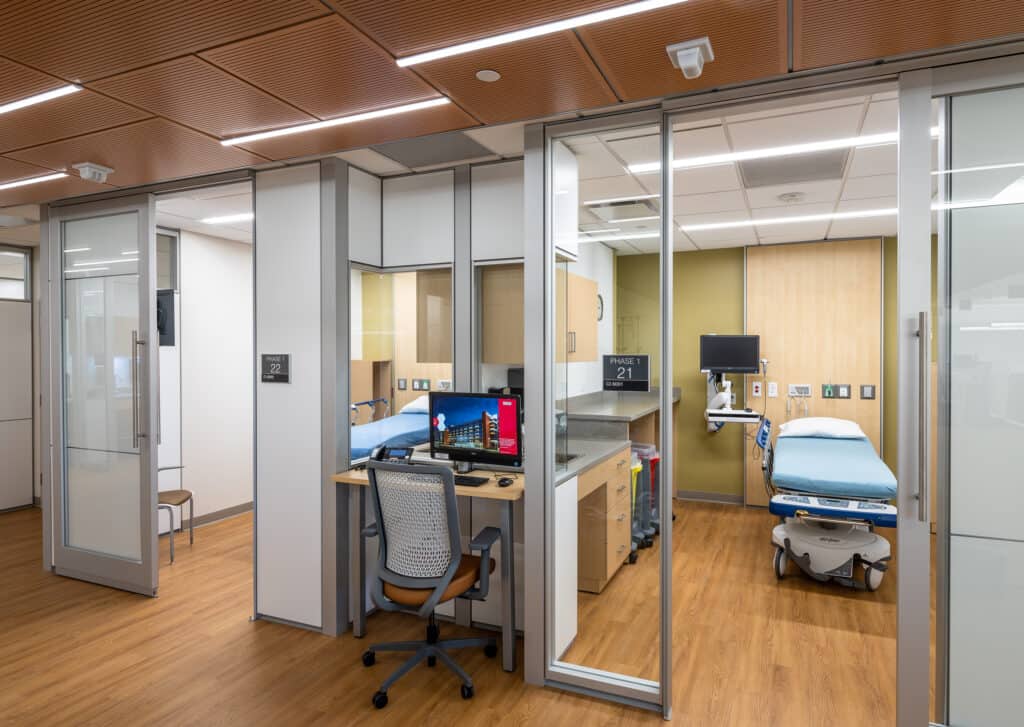
Recently Diagnosed Sister Joins Him - State-of-the-Art Infusion Center Opens at UAMS Winthrop P. Rockefeller Cancer Institute
- Myeloma Center Clinical Director, Research Staff Play Pivotal Role in Most Recent Discoveries in Castleman Disease
- Behind the Scenes
- Home Page of Magazine
2022
Editor
Linda Haymes
Creative Director
Mindy Stout
Photographer
Bryan Clifton
Evan Lewis
Director
UAMS Winthrop P. Rockefeller Cancer Institute
Michael Birrer, M.D., Ph.D.
Clinical Director
Myeloma Center
Frits van Rhee, M.D., Ph.D.
Director of research
Myeloma Center
Fenghuang (Frank) Zhan, M.D., Ph.D.
Chancellor
University of Arkansas for Medical Sciences
Cam Patterson, M.D., MBA
Myeloma is published once a year by the Myeloma Center, University of Arkansas for Medical Sciences